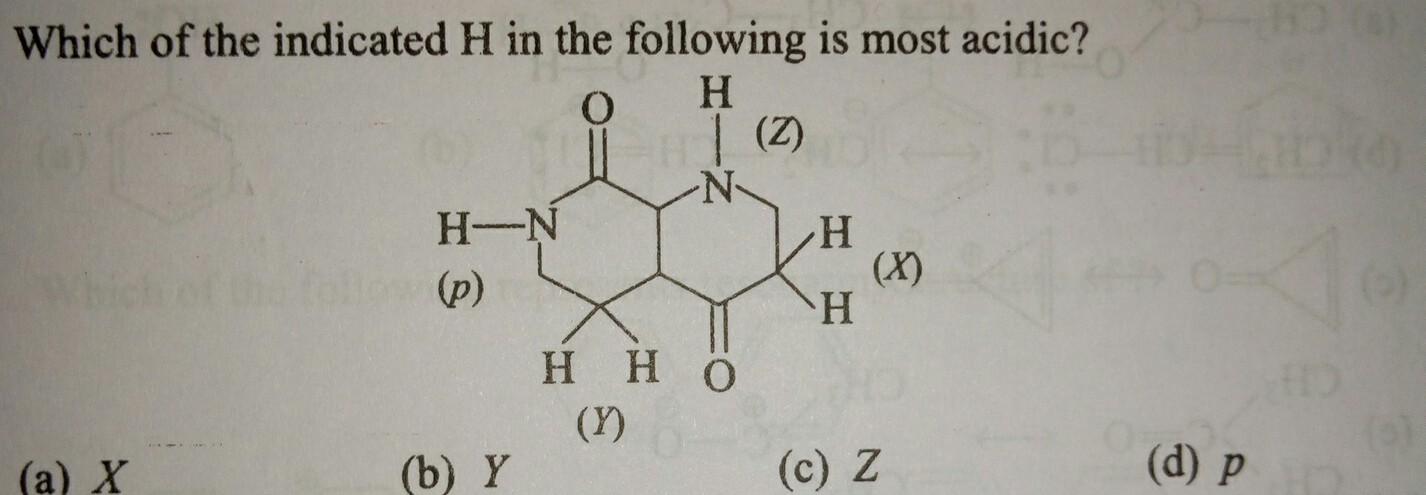
Find most acidic hydrogen?

2 Answers
Identify familiar functional groups.
#p# is an amide proton.#"pK"_a ~~ 15# #Y# is an alkyl proton para to a carbonyl.#"pK"_a > 50# #X# is an alkyl proton adjacent to a carbonyl.#"pK"_a ~~ 20# #Z# is an amine proton.#"pK"_a > 30#
Therefore,
http://cactus.dixie.edu/smblack/chem2310/summary_pages/pka_chart.pdf
The most acidic hydrogens are (d) p.
Explanation:
(b) Y
Protons Y are alkane hydrogens. They are the least acidic.
(c) Z
Protons Z are amine hydrogens.
They are slightly more acidic than alkanes because
(a) X
Protons X are alpha to a carbonyl group.
They are more acidic because the electrons of the conjugate base can be delocalized onto the adjacent carbonyl group to form a resonance-stabilized enolate ion.
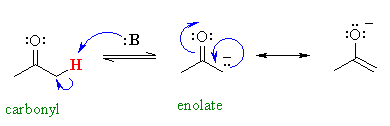
(d) p
Protons p are amide hydrogens.
The lone pair electrons can be delocalized onto the adjacent carbonyl group to form a resonance-stabilized anion.
Thus, protons p are the most acidic protons.


