How can I calculate equivalent hydrogens?
1 Answer
How can I calculate equivalent hydrogens?
One way is to replace each H with a different group and see if you get a different compound.
Example 1. Identify the equivalent hydrogens in 2-methylpropane.

If I replace any of the nine CH₃ atoms with a Cl, I'll always get the same compound: 1-chloro-2-methylpropane. So the nine CH₃ atoms form one set of nine equivalent hydrogen atoms.
If I replace the H atom on C-2, I'll always get a different compound: 2-chloro-2-methylpropane. So this lone H atom forms a second set consisting of 1 hydrogen atom by itself.
**Example 2. ** Identify the equivalent hydrogens in methyl acetate.
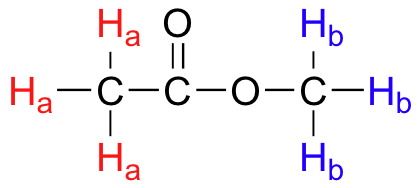
If I replace any of the red CH₃ atoms, I'll always get the same compound: methyl chloroacetate. So the red
If I replace any of the blue CH₃ atoms, I'll always get a different compound: chloromethyl acetate. So the blue
Now that you have the idea, try to identify the equivalent H atoms in ethyl acetate, CH₃COOCH₂CH₃, and the isomeric chloropropanes, C₃H₅Cl.

