How can I calculate the polarity of a compound?
1 Answer
LONG ANSWER. First, a distinction must be made between the polarity of a bond and the polarity of a molecule (or compound).
Bond polarity refers to a separation of electric charge that results from a difference in electronegativity between the two atoms or groups that bond together.
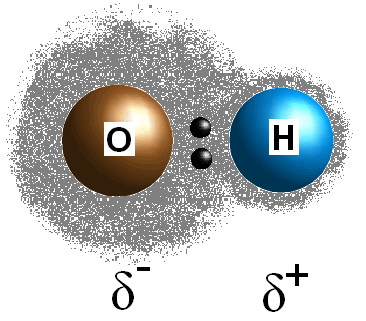
This difference in electronegativity values between the two atoms causes the bond's electrons to be shared unequally, thus creating a postive charge (
If the bond between the two atoms in not polar, i.e the difference in electronegativity between the two atoms is less than 0.5, then your molecule will be non-polar. If the bond is indeed polar, you can proceed to try and determine the polarity of the molecule.
A molecule's geometry is an important factor in determining the polarity of a molecule. These aforementioned partial charges give rise to a bond dipole moment,
If the orientations of these bond dipole moments cancel each other out, then the molecule is said to be non-polar. If however they do not, you are dealing with a polar molecule.
Some examples where bond dipole moments cancel each other out, resulting in a non-polar molecule:
 )
)
Dipole moments are drawn with the arrow pointing at the more electronegative atom and the plus side on the less electronegative atom. Notice that for
 )
)
The three dipole moments cancel each other out as a result of the symmetrical arrangement of the bonds (see more on vector addition).
An assymetrical arrangement of the partial charges results in a polar molecule, as you can see for water:
 )
)
The two dipole moments add to each other creating an overall dipole moment, and thus a polar molecule.
As a conclusion, in order to predict a molecule's polarity, you must be familiar with Lewis structures, electronegativity, VSEPR Theory, and bond polarity.

