How can I identify alkenes?
1 Answer
May 23, 2014
Alkenes are hydrocarbons that possess a double bond and have the general formula
For example if we look at the simplest alkene - ethene, for example - the formula is
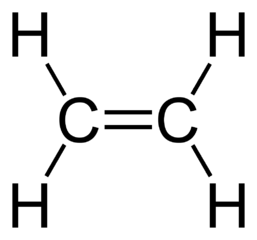
Notice again that the number of carbons is 2, so our n value would be 2. This results in a 2n value of 4, which corresponds to the number of hydrogens here.
It is possible for compounds to have multiple double bonds, and the general formula for these will be represented as

