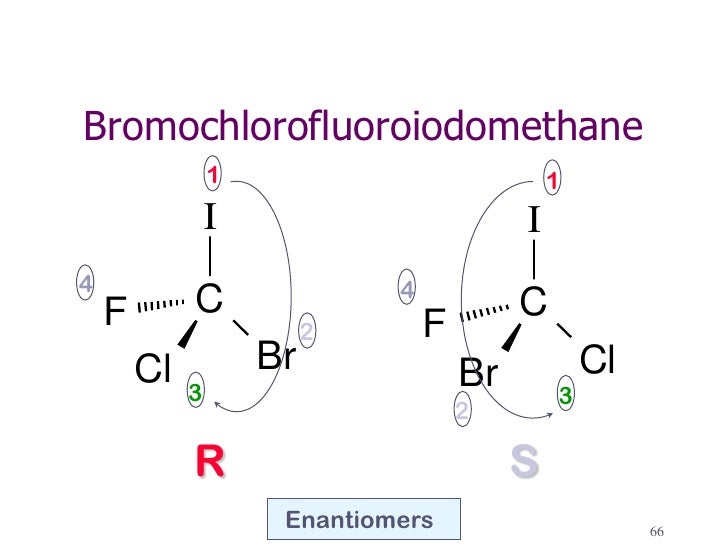
How can I show the R-configuration of the molecule bromochlorofluoroiodomethane?
1 Answer
Here's how I do it.
Step 1. Draw a C atom with a wedge, a dash, and two solid bonds.
Step 2. Add F, Cl, Br, and I to the bonds in any order.
We have a 50:50 chance of getting it right. Here's one possibility.

Step 3. Assign priorities to the groups.
I = 1; Br = 2; Cl = 3; F = 4.
Step 4. Assign stereochemistry
F has the lowest priority. In this drawing, it is more convenient to view the molecule from above.
Then I → Br→ Cl = 1 → 2 → 3 goes in a counterclockwise direction (
But we are viewing the molecule with our eye closest to the low-priority group.
So this is really (
If we had gotten it wrong, we would have interchanged any two of the groups.
For example, in the diagram below, interchanging the Cl and Br atoms converts the