How can nuclear fission produce energy?
1 Answer
A nuclear fission produces nuclei that have less mass than the original nucleus, and the excess energy is released.
The mass of a nucleus is always less than the mass if its protons and neutrons. For example, the total mass a carbon-12 atom is 0.0989 u less than the total mass of its separate protons, neutrons, and electrons.
The difference in mass is called the mass defect and is converted to binding energy according to the formula E = mc². The binding energy of carbon-12 is 92.15 MeV (mega-electron volts).
The atomic nuclei with the highest binding energies per nucleon are around the size of Fe-56 and Ni-62. They are among the most stable of nuclei. Heavier nuclei have lower binding energies.
Consider the fission reaction
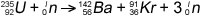
The binding energies are:
U-235 = 1784 MeV
Ba-142 = 1180 MeV
Kr-91 = 778 MeV
Binding energy of products – binding energy of reactants =
(1180+ 778 - 1784) MeV = 174 MeV per nucleus of U-235.
The difference, 174 MeV, is the energy released in the fission process. It is equivalent to about 4 kilotons of TNT per mole of U-235.

