How can we explain why electrons don't spiral into the attracting nucleus?
2 Answers
That is the "energy" of the electron. There is no opposing force.
Explanation:
Essentially, at the atomic scale, the electron is orbiting in a vacuum. With no "drag" there is nothing to reduce its angular momentum around the nucleus.
Here's how I would explain it.
Explanation:
This is exactly what classical physics predicts.
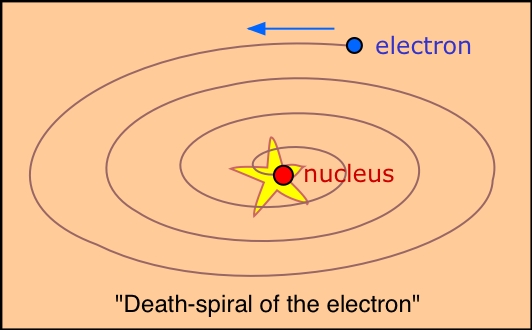
However, we now know that electrons are governed by the laws of quantum mechanics.
Electrons don't really orbit a nucleus.
Instead, we must think of the electron as a cloud of electron density.
The electron could be anywhere in a spherical "shell" around the nucleus.
We could think of the atom as consisting of many thin shells with radius
(From Chemistry LibreTexts)
For each shell, the area is
The probability density is greatest at
As we move outwards, the area
However, at large distances from the nucleus, the probability density is quite small, so the probability
Also, close to the nucleus, the volume of each shell is small, so the probability
So, the highest probability of finding the electron is going to be where the volume of the shell is big enough that
Thus, a plot of radial probability has a maximum at a certain distance from the nucleus [graph (d) above].
Most important, the probability of finding an electron at the nucleus is zero.


