How can we find the number of protons and electrons of any element?
1 Answer
The numbers of protons and of electrons of any element are the same as its atomic number.
Explanation:
The atomic number is the number of protons in the nucleus of an atom. The Periodic Table arranges the elements in order of their atomic numbers.
We use the Periodic Table to find the atomic number of the element. The atomic number is in the upper left corner.
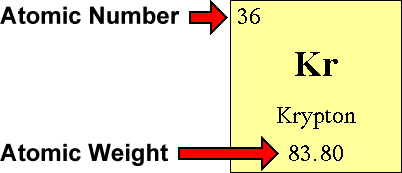
For example, the atomic number of krypton is 36. This tells us that an atom of krypton has 36 protons in its nucleus.
By definition, atoms have no electrical charge. The positive charges of the protons must balance the negative charges of the electrons.
Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons as well as 36 protons.
For any element:
Number of Protons = Number of Electrons = Atomic Number
EXAMPLE:
How many protons and electrons are in an atom of gold?
Solution:

Gold has atomic number 79. Thus, gold has 79 protons and 79 electrons.

