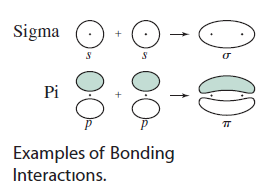
How can you find a sigma bond in an atom?
1 Answer
Jul 28, 2016
You can't. Atoms don't have sigma bonds. They are alone.
Sigma bonds require head-on overlap of
These atoms might be the same kind of element, but there must be at least two atoms for there to be a single bond, and thus, a sigma bond.