How did Arrhenius describe acids?
1 Answer
Jul 17, 2018
Substances that produce hydrogen ions in aqueous solution
Explanation:
Arrhenius defines acid as substance that produces hydrogen ions
Here's the general equation defining Arrhenius acid and base:
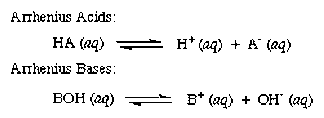
Notice the acid, HA, will ionize in water to produce hydrogen ion
Common examples of Arrhenius acids are:
- nitric acid,
#HNO_3# - hydrochloric acid,
#HCl# - sulfuric acid,
#H_2SO_4# - acetic acid,
#HCH_3CO_2#

