How does molality affect the boiling point?
1 Answer
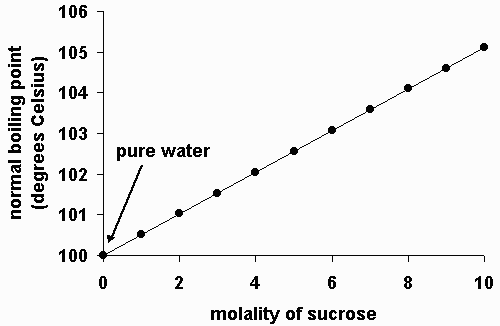
Whenever a non-volatile substance is dissolved in a solvent, the boiling point of the solvent increases. The higher the concentration (molality), the higher the boiling point.
You can think of this effect as dissolved solute crowding out solvent molecules at the surface, where boiling occurs. The higher the concentration of solute, the more difficult it is for solvent molecules to escape into the gas phase. However, the rate of condensation from the gas to the liquid is essentially unaffected. Therefore, it requires a higher temperature for enough solvent molecules to escape to continue boiling at atmospheric pressure. Thus, the boiling point is elevated.
To a fair approximation, the amount by which the boiling point is raised is linearly dependent on the molality of the solute. And to a fair approximation, it doesn't matter what type of solute you use; the only thing that matters is the concentration of dissolved solute molecules or ions.