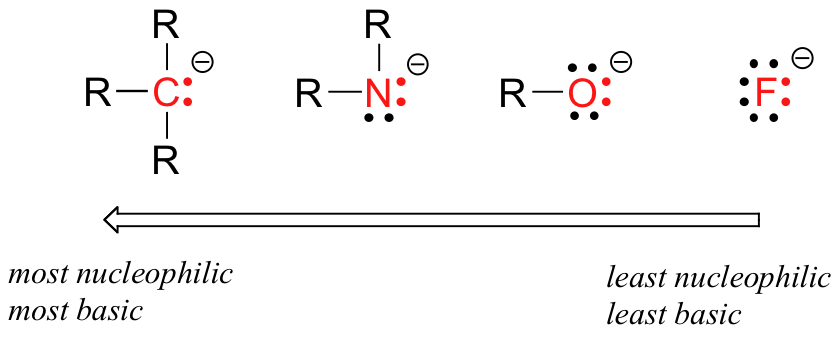
How does the reactivity of a nucleophile change across a row in the periodic table?
1 Answer
Jan 3, 2015
The reactivity of a nucleophile decreases from left to right in a row across the Periodic Table.
A nucleophile is a Lewis base that donates an electron pair to an atom other than hydrogen. In organic chemistry, this usually means a carbon atom.

Electronegativity increases from left to right across the Periodic Table.
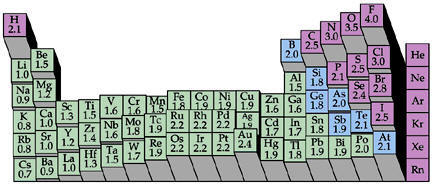
This means that atoms on the right hand side are less likely to donate their electrons to form bonds with other atoms.
Their nucleophilicity decreases from left to right.