How does the value of an equilibrium constant relate to the relative quantities of reactants and products at equilibrium?
1 Answer
Apr 19, 2015
The Equilibrium constant is directly proportional to the products of a reaction and inversely proportional to the reactants.
I say this because as you know the Equilibrium Constant is concentration of the products raised to the power of number of moles divide by the concentration of the reactants raised to the power of number of moles. (Liquids and solids don't take part in the constant)
Image from: 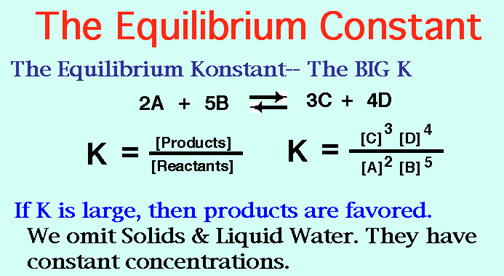
Hope I helped :)

