How many C-C bonds are there in Hexene? What is the structural formula for Hexene?
2 Answers
"Hex" is the prefix for 6 carbon atoms and "ene" is the suffix for an alkene.
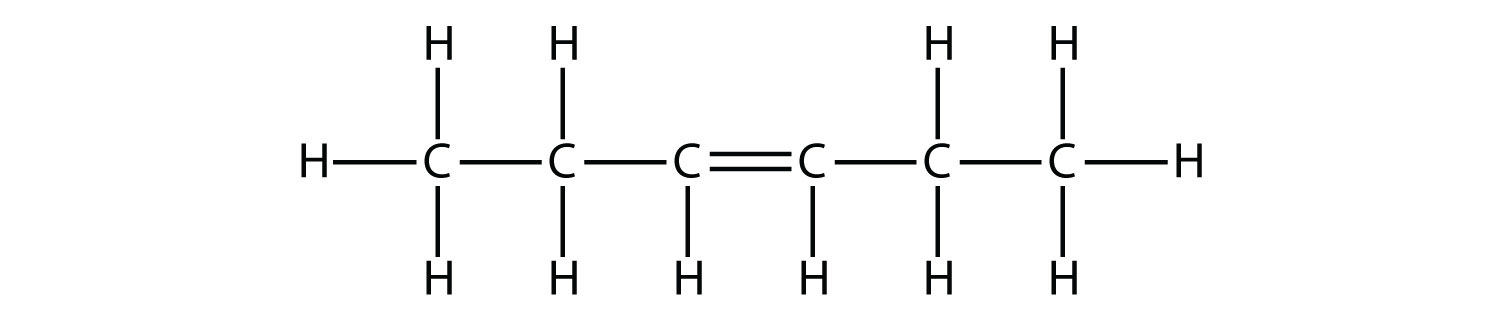
So there are 4 C-C bonds and 1 C=C bond
The suffix -ene means that a hydrocarbon molecule has at least one pair of double-bonded carbon atoms,
The chemical formula for hexene is
There are several structural isomers of hexene based on the location of the double-bonded carbon atoms, and other factors.
The following diagram is 1-hexene, a hexene structural isomer with the double bond between carbon atoms 1 and 2.
 )
)
The following hexene isomer is named 3-hexene because the double bond is between carbon atoms 3 and 4.
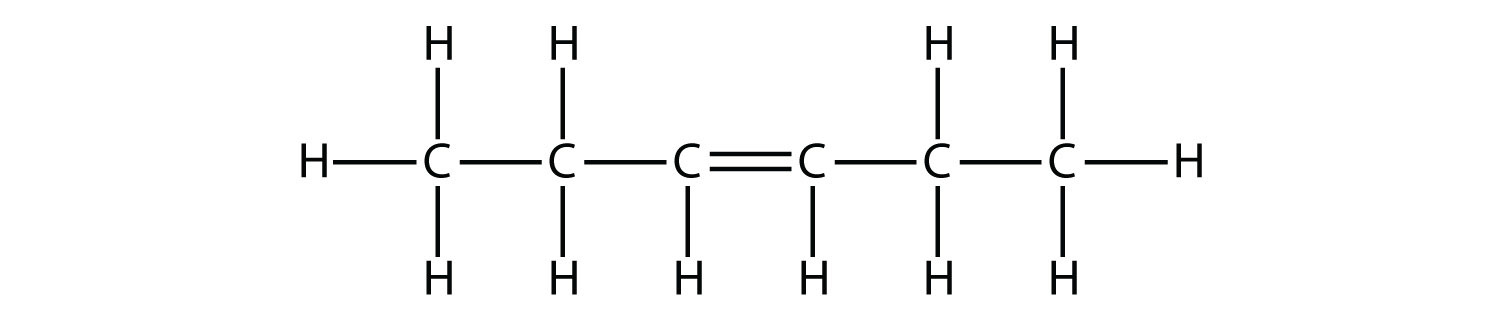
The following hexene isomers are called cis-3-hexene and trans-3-hexene. These are 3-hexene isomers that differ in three-dimensional space. Notice that the
 )
)



