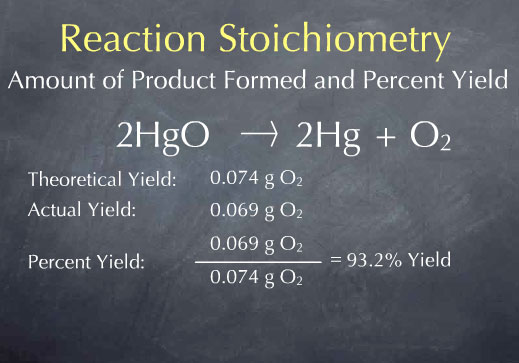
If the reaction of 0.112 grams of H2 produces 0.745 grams of H2O, what is the percent yield? Fe3O4+4H2=>3Fe+4H2O
1 Answer
Sep 18, 2014
The percent yield is 74.4 %.
Fe₃O₄ + 4H₂ → 3Fe + 4H₂O
First, calculate the theoretical yield of H₂O.
Theor. yield =
Now calculate the percent yield.
% yield =