In the preparation of an ester by refluxing with ethanol and sulfuric acid, the mixture is poured into water. What would you do next?
This is a question we got after doing a lab on seperating organic compound using a seperating funnel so maybe it has something to do with that? I'm so lost and help would be great.
This is a question we got after doing a lab on seperating organic compound using a seperating funnel so maybe it has something to do with that? I'm so lost and help would be great.
1 Answer
Nov 12, 2017
It depends on the ester.
Explanation:
If the ester is a solid
Remove it from the water by suction filtration and purify it by recrystallization from a suitable solvent.
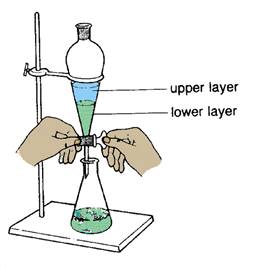
Dry your combined ether extracts over an appropriate drying agent.
Next, remove the ether by distillation. Then raise the temperature and continue to purify the ester by ordinary distillation or vacuum distillation.

