Is CH3CO2H a dipole dipole?
1 Answer
The intermolecular forces in CH₃CO₂H are an especially strong type of dipole-dipole force given its own special name — hydrogen bonding.
Hydrogen bonds form when you have a negative O, N, or F atom in one molecule and a positive H atom attached to an O, N, or F atom in another molecule.
Water has strong hydrogen bonds.

Like water, acetic acid has strong hydrogen bonds. In solid acetic acid, the molecules form cyclic pairs connected by hydrogen bonds.
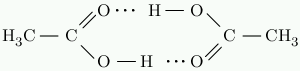
Acetic acid melts at 16 °C. Butane, C₄H₁₀, has almost the same molar mass. It melts at -140 °C. The hydrogen bonds cause this enormous difference in melting point.


