Is H2CO3 a polar molecule?
1 Answer
Aug 22, 2015
Yes,
Explanation:
The Lewis structure of
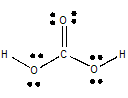
(from employees.csbsju.edu)
With respect to the carbon atom, this is an
The geometry about the
So the molecule is polar.

