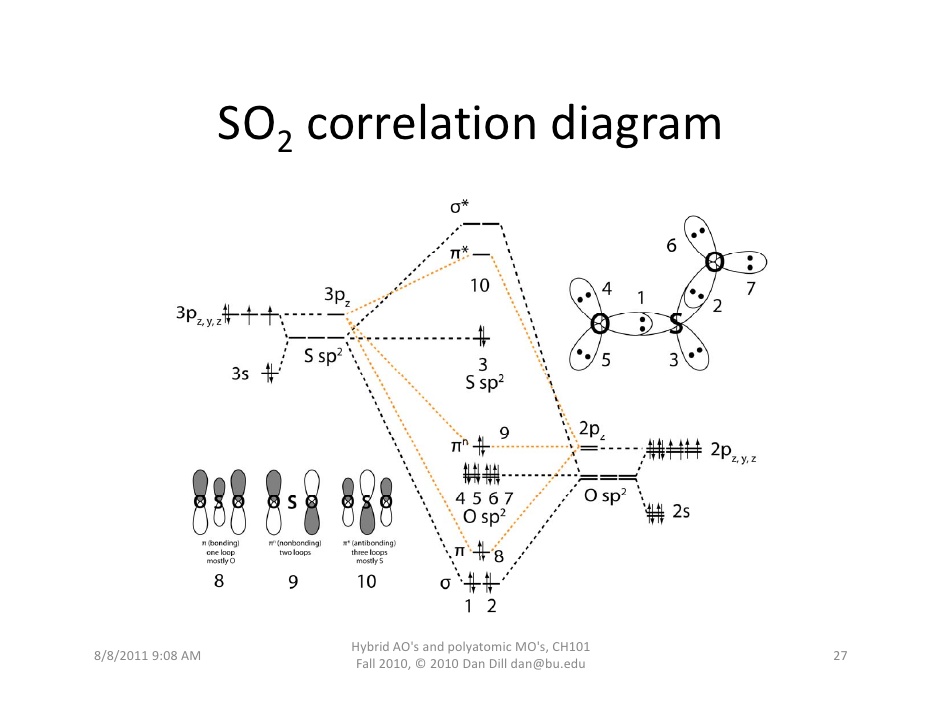
Yes. You can see a fuller discussion here, but the gist of it is that #"SO"_2# has a #pi^"*"# antibonding molecular orbital formed by the overlap of the oxygen #2p_z# and sulfur #3p_z# atomic orbitals.
 )
)
That #pi^"*"#, labeled MO 10, acts as the lowest-unoccupied molecular orbital (LUMO), which can accept electrons.
Since it is closest in energy to sulfur's #3p_z# orbital, the nucleophilic attack will effectively (visually, in a drawn-out reaction mechanism) donate two electrons into sulfur's #3p_z# atomic orbital.
Therefore, as an electron pair (#pi#) acceptor, by definition, #"SO"_2# it can be a Lewis acid.
 )
) 
