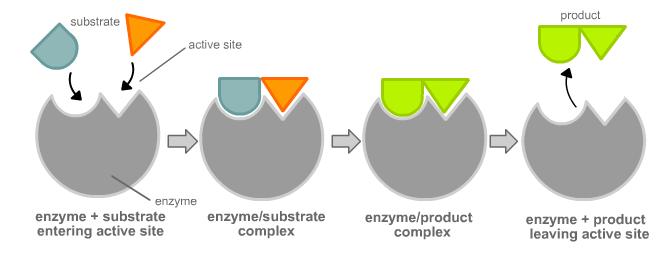
This illustrations depicts the #color(magenta)"lock and key model"# of enzyme and substrate binding first proposed by Emile Fischer in his studies on enzymes in the nineteenth century.
#ul"Illustration going from righ t to left"#
#1. "Arrows point to the active sites"#
#"of the enzyme."#
#2. "Substrates bind to the active"#
#"sites of the enzyme -- [enzyme-substract] complex forms"#
#3. "Enzyme-substracts turns into"#
#"enzyme-products ."#
#4. "Products are released after catalyzed"#
#"reaction."#
#color(white)(aaaaaaaa)"Enzyme remains unchanged."#
#---------------#
This model proposes the idea that enzymes are like locks and substrates are the keys to that lock. The key has to match up perfectly to the grooves of the lock, or in other words, be #color(magenta)"complementary"# to them.
 )
)
This model, however, posed many problems to scientists because such an #color(magenta)"enzyme-substrate complex"# would lead to poor catalysis. The reason being is after binding, the enzyme-substrate complex, [ES] would become more stable than the substrate itself, [S], and thus, be lower in free energy, #"G"#. And because [ES] is lower in free energy, the activation energy, symboled as #Delta"G"^‡"#, would increase instead of decrease.
#color(white)(.......)color(red)[Delta"G"^‡"catalyzed" >DeltaG^‡"uncatalyzed"#
![enter image source here]()
In later studies, biochemists came up with a better model to explain how enzymes and substrates bind - #color(magenta)["the induced fit model"#.
 )
)

