The following reaction was monitored as a function of time: #AB -> A + B#. A plot of #1/[AB]# versus time yields a straight line with slope #5.6xx10^(-2) (M.s)^-1#. What is the value of the rate constant (k) for this reaction temperature?
1 Answer
Jul 9, 2016
Explanation:
This looks like a 2nd order reaction:
For which the rate of disappearance of
Integrating this gives:
Where
This means a plot of
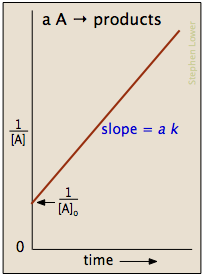
The graph is a straight line of the form

