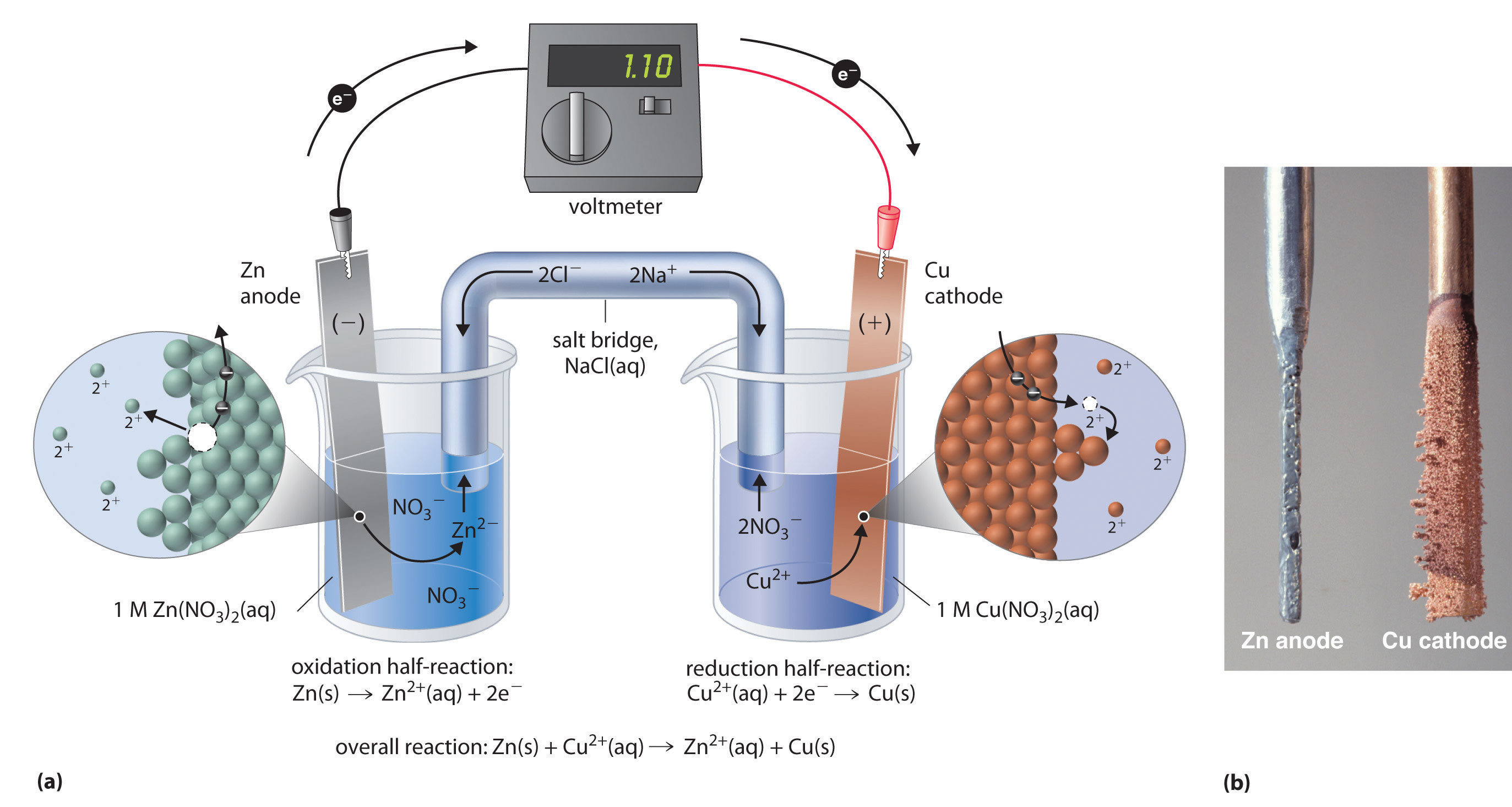
Two half-cells in a galvanic cell consist of one iron (Fe(s)) electrode in a solution of iron (II) sulphate (FeSO4(aq)) and a silver (Ag(s)) electrode in a silver nitrate solution. What will happen to the mass of the cathode and the mass of the anode while the cell is operating?
1 Answer
The mass of the cathode increases, and the mass of the anode decreases, while the cell is operating.
To solve this problem, you need the reduction potentials for iron and silver:
Fe²⁺(aq) + 2e⁻ → Fe(s); E₀ = -0.44V
Ag⁺(aq) + e⁻ → Ag(s); E₀ = +0.80
We must combine these half-reactions to get a positive cell potential. we reverse the Fe half-reaction.
Fe(s) → Fe²⁺(aq) + 2e⁻; E₀ = +0.44 V
2×[Ag⁺(aq) + e⁻ → Ag(s)]; E₀ = +0.80 V
Fe(s) + 2Ag⁺(aq) → Fe²⁺(aq) + 2Ag(s); E₀ = +1.24 V
This tells us that Fe is spontaneously oxidized to Fe²⁺(at the anode).
The solid Fe anode gradually disappears as it is converted to the soluble Fe²⁺ ions. The mass of the anode decreases during the cell operation.
The Ag⁺ ions are spontaneously reduced to solid silver (at the cathode). They stick to the cathode, so the mass of the cathode increases during the cell operation.
In any galvanic cell with reactive electrodes, the mass of the cathode increases and the mass of the anode decreases while the cell is operating.