The #sp^3# orbitals are formed from 1 s and 3 p orbitals. The energies of the there four orbitals are generally averaged forming four equal orbitals. These orbitals are spread out uniformly in space forming a tetrahedral geometry. The tetrahedral shape allows the easy formation of direct sharing and overlap of electron density for sigma bonds and double pi bonds.
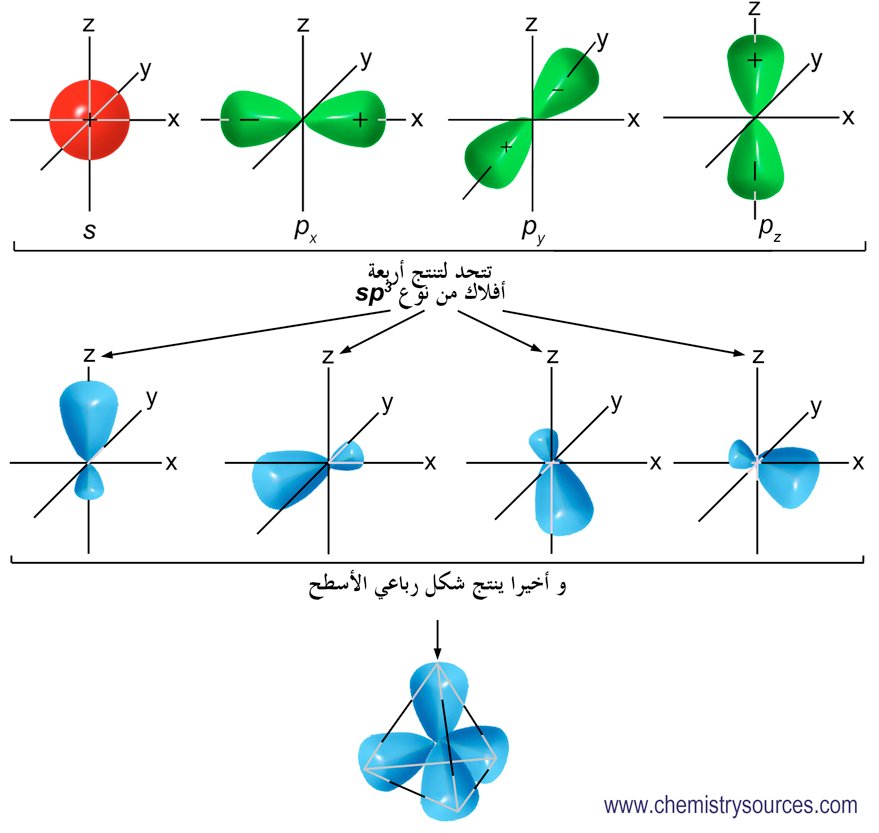
#sp^3# orbitals allows elements like carbon to spread the valance electrons out over 4 equal orbitals to increase bonding. Carbon that has hybridized forms 4 bonds. Carbon that has not hybridized can only form 2 bonds as in Carbon monoxide. This is because there are two paired electrons in the 2s orbital. Paired electrons do not easily form bonds.

