What are the possible resonance structures of #CH_3-CH=CH-CH=O# and #CH_3-COO^-#? What is the order of stability of the contributing resonance structures?
2 Answers
See below.
Explanation:
Just remember that the electrons will move to the more electronegative atom or the more positive atom.
(a) But-2en-al
There is no positive atom, but
Now the carbon atom has a
Structure
Structures
(b) Acetate ion
Here again, there is no positive atom, but the other
Let's move the electrons toward it.
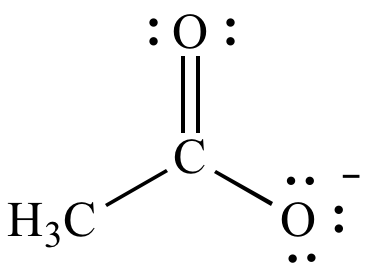
You can also move the electrons in the carbonyl group to the carbonyl
(from www.chem.ucla.edu)
Of the three structures above, the first two are equivalent and of equal energy.
The third structure is a minor contributor because it has three charges instead of one,
Although Ernest provided an attentive, helpful answer, I have provided the bond-line structures for the resonance structures, below.
For the first molecule, there aren't many resonance structures that adhere to the octet rule besides the one we start with,





The electrons move to the right with each resonance structure. The first is major, and the rest our minor due to its octet rule satisfaction.
For acetate, I think Ernest did a fine job that doesn't need any more detail.


