What are the resonance structures for #SO_2#?
1 Answer
Sulfur dioxide, or
Let's draw the first two Lewis structures for
The first two Lewis structures that can be drawn for
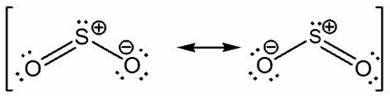
All 18 valence electrons are accounted for - 6 electrons from 3 bonds and 10 electrons distributed as lone pairs on the three atoms. A couple of important things to notice here.
These two resonance structures are equivalent and will contribute equally to the hybrid structure. Both structures have formal charges - the negative formal charge is placed on the more electronegative atom - oxygen, while the positive charge is placed on sulfur, the less electronegative of the two atoms.
In this case, the actual structure would be a hybrid that would look like this
The negative charge will be split on the two oxygen atoms. The charges on the atoms are
Another Lewis structure that can be drawn for
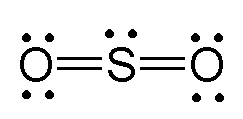
This time no formal charges are present - each oxygen atom needs 6 electrons and gets 6 electrons, the same being true for sulfur. Since sulfur has access to its 3d-orbitals, it's perfectly capable of expanding its octet to accomodate 10 electrons instead of 8.
So, in theory, this structure would be more stable than the previous two based on the fact that it has more covalent bonds and no formal charges an any of the atoms.
However, like I've said, experimental data would point towards the hybrid model; partial charges on all atoms would be present, and the molecule's double bonds have single bond character as well.
Moreover, the molecule's bond order, which refers to the number of bonds between a pair of atoms, is
So, as a conclusion, all three structures are valid; the one with two double bonds is preferable from a theoretical point of view, while the other two come closer to what experimental data show for the

