What does the ideal gas law allow a scientist to calculate that the other gas laws do not?
1 Answer
May 26, 2015
Quick answer: nothing.
There are four gas laws.
Volume is inversely proportional to pressure, or
Volume is directly proportional to temperature, or
Gay-Lussac's or Amonton's Law
Pressure is directly proportional to temperature, or
Volume is directly proportional to the number of moles, or
Put them all together, and you get
The Ideal Gas Law
We usually replace the constant
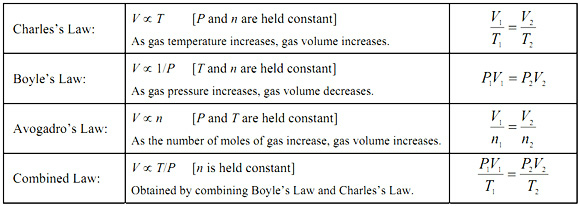
The Ideal Gas Law is simply a combination of the other four gas laws.

