What intermolecular forces are present in #NH_3#?
2 Answers
Dispersion forces and hydrogen bonding....
Explanation:
And of course, the most significant intermolecular force is hydrogen bonding. The normal boiling point of ammonia is
Again, as nitrogen, and oxygen, are the most electronegative elements of their Groups, the dipoles they form in their bonds with hydrogen are the MOST polar, and thus give rise to the greater intermolecular force, and therefore the greater boiling points...
I'm assuming you mean inter-molecular forces...
Explanation:
The following image illustrates the hydrogen-bonding intermolecular forces of attraction when

The next image illustrates the weak electromagnetic intermolecular forces of attraction when
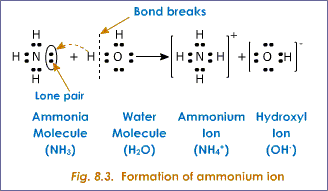
I hope this helps!