What is line emission spectrum?
1 Answer
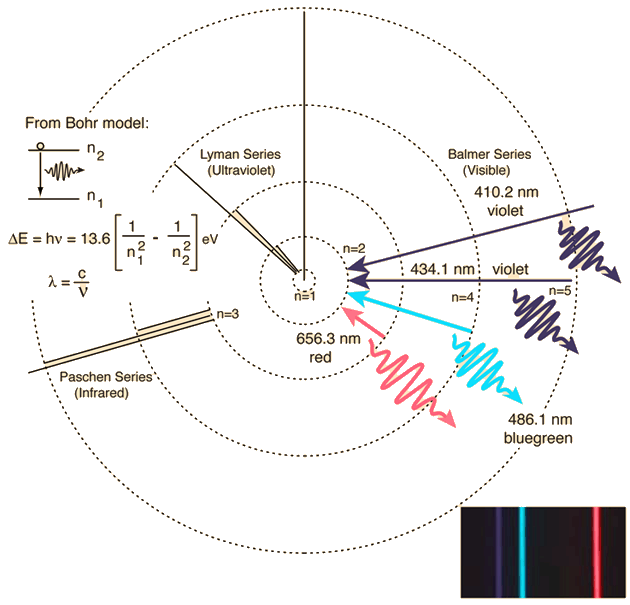
It is a sequence of lines of different color/frequency that represents a kind of "photograph" of the structure of an atom.
Explanation:
In a very simplistic way you can consider a gas of atoms of a substance illuminated by light. This means that the gas is receiving energy carried by the photons of light (as in
After a short while the atoms emit the surplus energy (Emission) and we can "see" this emission as photons of light of frequency
As a real example you have Hydrogen that in emission shows colors corresponding to various transitions: