What is the cause of the color change observed when #HCl# is added to the complex ion #[Cu(NH_3)_4]^(2+)#?
1 Answer
Jul 18, 2017
I have explained a similar ligand displacement reaction to this in the answer below:
Explanation:
https://socratic.org/questions/hydrochloric-acid-copper-ll#415291
In this case the complex we have is tetraammine copper(II):


If excess chloride ions are added the ammonia ligands are displaced and the green, tetrachloro copper(II) complex forms:
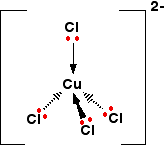
The solution looks like this:


