What is the change in atomic mass number when an atom emits an alpha particle?
1 Answer
Oct 30, 2015
The atomic number will be reduced by two, which means the element transforms into a different element.
Explanation:
An alpha particle is essentially a helium nucleus composed of two protons and neutrons.
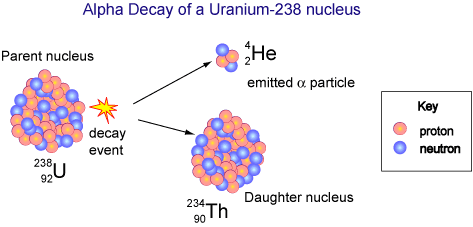
Since atomic number is the number of protons, if an atom emits an alpha particle, its atomic number will be reduced by two, and its mass number will be reduced by four. The atom will have undergone transmutation into a different element. This is called alpha decay.
In the diagram below, a uranium atom with atomic number 92 undergoes alpha decay and transforms into a thorium atom with atomic number 90. Notice also that the mass number has decreased by four.