What is the most stable carbocation?
1 Answer
The tricyclopropropylcyclopropenium cation is the most stable carbocation.
Explanation:
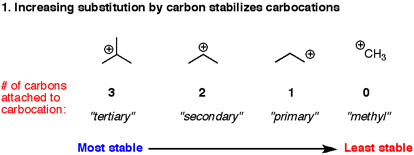
1° < 2° < 3°
We know that the stability order of aliphatic cations is
Resonance Stabilization
Neighboring double bonds are better stabilizers, because they can donate electrons by resonance.
Hence, the triphenylmethyl cation,
However, the resonance overlap of the π orbitals is not perfect because steric hindrance forces the ion to have a propeller shape.
(Adapted from Schoolbag.info)
Tricyclopropylmethyl
More stable is the tricyclopropylmethyl cation.
The bent bonds of the cyclopropane ring can overlap with the empty
(Adapted from Wikipedia)
Arenium ions
Nothing beats the stability of aromatic cations like cyclopropenium and cycloheptatrienylium (tropylium) cations.
(Adapted from www1.biologie.uni-hamburg.de)
The most stable carbocation
Add the conjugation with cyclopropyl rings, and the most stable carbocation prepared to date is probably the tricyclopropylcyclopropenium cation.


