What is the nuclear equation for the electron capture decay of Ar37?
1 Answer
The nuclear equation for the electron capture decay of Argon-37 is
During electron capture, an inner-orbital electron is captured by the nucleus, which results in the formation of a neutron after said electron combines with a proton.
Notice that the atomic number was reduced by 1 (
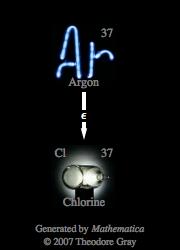
Argon-37 is one of the 24 isotopes of Argon and has a nuclear half-life of 35.04 days.


