What is the number of core and valence electrons for I?
2 Answers
Explanation:
Iodine,
That means that a neutral iodine atom will have a total of
Now, out of these
#n_"total" = n_"core" + n_"valence"#
But how would you distinguish between the two types of electrons?
To do that, you need to look at the electron configuration of iodine, which looks like this
#"I: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 5s^2 4d^10 5p^5#
You know that the valence electrons are defined as being the electrons located on the highest energy level of that atom, which is given by the principal quantum number,
Notice that, in iodine's case, the highest energy level is
This means that iodine has a total of
#underbrace(2e^(-))_(color(blue)("from 5s subshell")) + overbrace(5e^(-))^(color(red)("from 5p subshell")) = "7 valence " e^(-)#
The rest of the electrons will thus be core electrons.
#n_"core" = n_"total" - n_"valence"#
#n_"core" = 53 - 7 = color(green)("46 core "e^(-))#
Therefore, a neutral iodine atom has
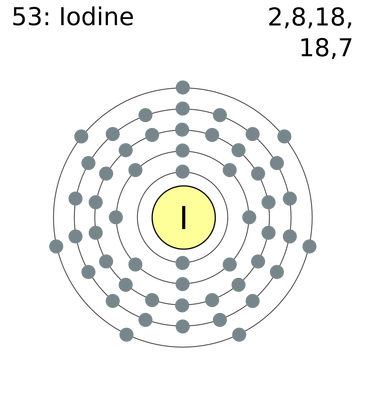
Iodine has
Explanation:
Iodine is in the group 17 elements, known as halogens, and all of them have
A valence electron is capable of bonding with valence electrons of another atom to complete the octet of electrons each atom needs in its outer shell.
The core electrons are not available for bonding with other elements
When more than one atom bonds with others, a molecule is formed. The completed molecule will not have an electron charge associated with it.

