What is the volume occupied by #3.0*10^23# molecules of bromine gas at STP?
1 Answer
Explanation:
 )
)
At standard temperature and pressure, the temperature is 273K and the pressure is 1 atm.
Next, list your known and unknown variables. Our only unknown is the volume of
We don't necessarily have 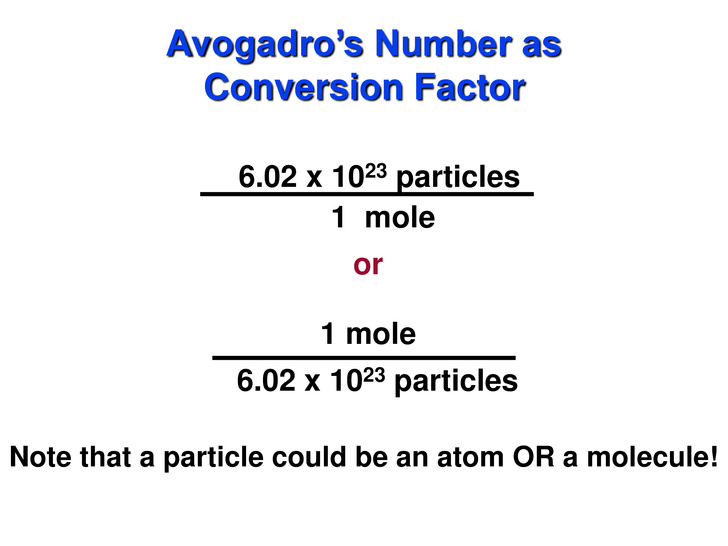 )
)
We'll use the second conversion factor because we can cancel out molecules and end up with moles:
Now all of the variables have good units! All that's left to do is rearrange the equation and solve for V like so:

