What volume of 0.10 M #NaOH# can be prepared from 250 mL of 0.30 M #NaOH#?
1 Answer
Explanation:
The thing to notice here is that the initial solution is
#(0.30 color(red)(cancel(color(black)("M"))))/(0.10color(red)(cancel(color(black)("M")))) = 3#
This tells you that the volume of the target solution must be
That is the case because when you dilute a solution, you decrease its concentration by increasing its volume while keeping the number of moles of solute constant.
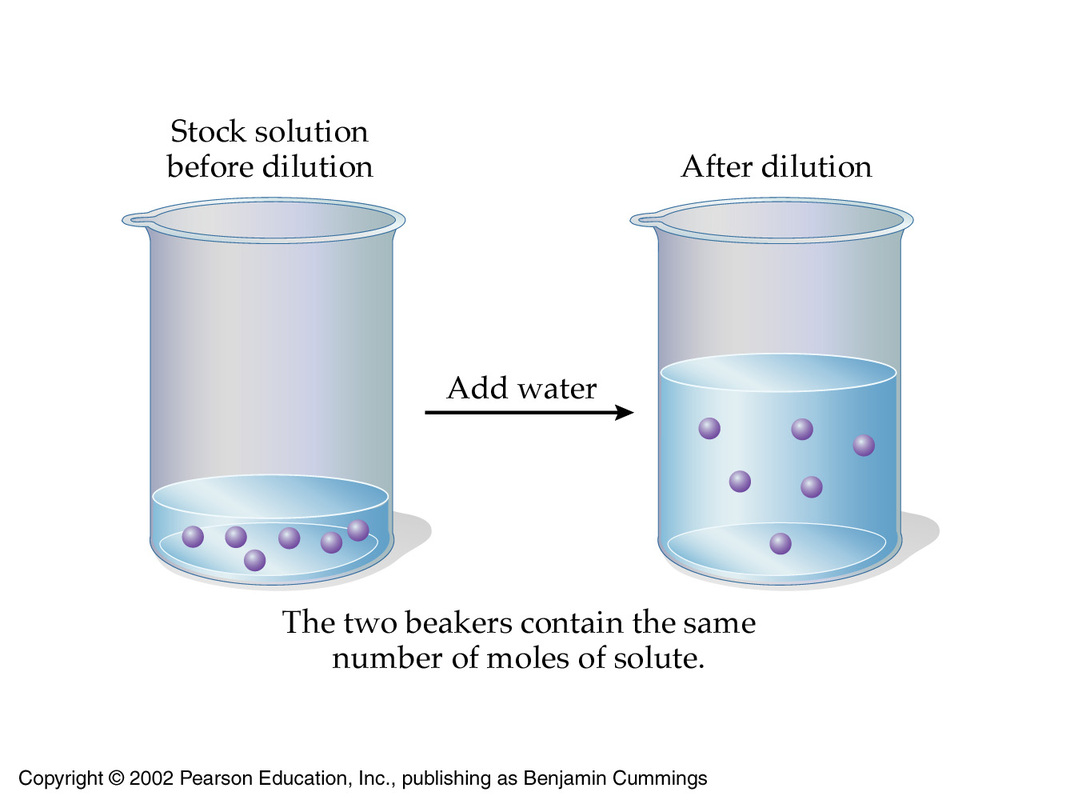
This implies that when the concentration of a solution decreases by a factor, which is usually called dilution factor,
#color(blue)(|bar(ul(color(white)(a/a)"DF" = V_"final"/V_"initial" = c_"initial"/c_"final"color(white)(a/a)|)))#
You thus have
#V_"final" = 3 xx "250 mL" = color(green)(|bar(ul(color(white)(a/a)color(black)("750 mL")color(white)(a/a)|)))#
The answer is rounded to two sig figs.
So, you can decrease the concentration of

