Why are s orbitals non directional?
1 Answer
s orbitals are nondirectional because they have spherical symmetry.
Explanation:
s orbitals are nondirectional because they have spherical symmetry.
Spherical symmetry means that the probability of finding an electron at a particular distance from the nucleus is same in all directions.
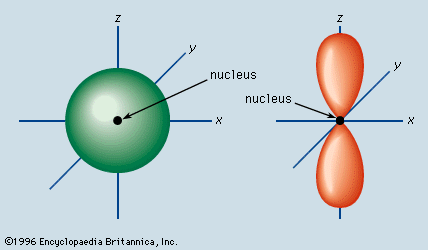
Compare that with a p orbital, which has a shape somewhat like a dumbbell. In a p orbital, the probability of finding an electron at a particular distance is greatest along the x, y, or z axis.
A p orbital is directional. An s orbital is nondirectional.

