Why can an anti-markovnikov radical addition of haloalkane only happen in presence of hydrogen peroxide?
1 Answer
Jun 13, 2015
In a "normal" Markovnikov attrition of
Explanation:

In the peroxide-catalyzed addition, the bromine radical adds to the carbon with more hydrogen atoms in order to form the more stable radical.
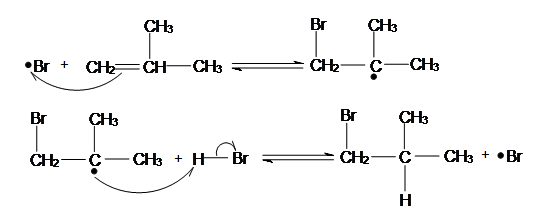
That means that the

