Why do primary alkyl halides generally undergo SN2 mechanisms?
1 Answer
Primary alkyl halides undergo
Explanation:
Steric Hindrance
As you add more alkyl groups o the α carbon atom, the substrate becomes less susceptible to
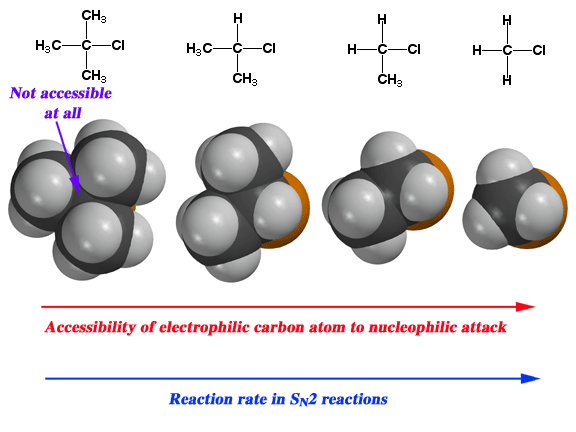
A 1° alkyl is sterically unhindered, so an
Instability of 1° Carbocations
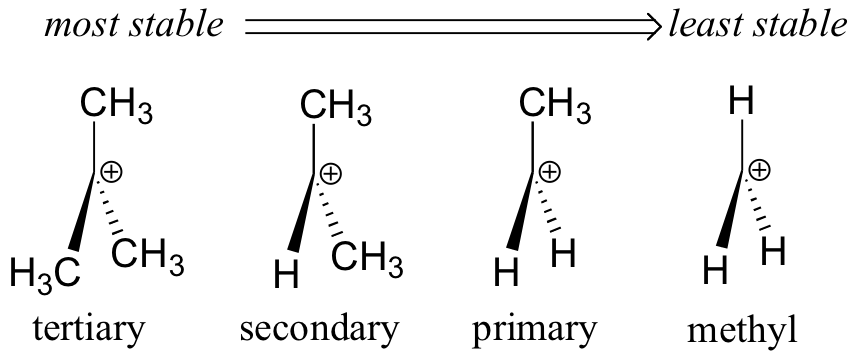
Alkyl groups release electrons by inductive and hyperconjugation effects so that they can stabilize a positive charge on the α carbon.
A 1° alkyl halide has only one alkyl group, so it is relatively unstable. It is unlikely to form a 1° carbocation in an
Instead, it will take the lower-energy

