Why does methanol (an alcohol) make methyl orange (an indicator) turn yellow?
1 Answer
Apr 20, 2016
See the explanation below.
Explanation:
Methyl orange is an intensely-coloured indicator that is red below
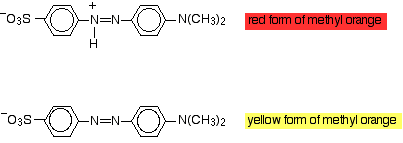
The red (acid) form has an
The yellow (basic) form has lost the
The
Methanol (
Methanol is even less able than water to protonate the

