Why is CO a lewis acid?
1 Answer
Jan 16, 2016
Explanation:
The antibonding orbital looks like the image below.
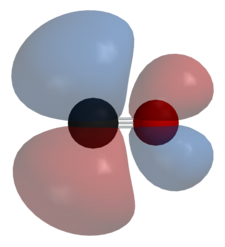
An example is nickel tetracarbonyl,

Because the carbon monoxide is accepting electron density, it is behaving as a Lewis acid.

