Why is #SCl_2# polar?
I have the Lewis structure and from there I can see that it has polar bonds because of the Cl, so both S - Cl bonds are polar bonds. But shouldn't the bonds cancel each other out?
I have the Lewis structure and from there I can see that it has polar bonds because of the Cl, so both S - Cl bonds are polar bonds. But shouldn't the bonds cancel each other out?
1 Answer
Because of the lone pairs of electrons present on the sulfur atom.
Explanation:
The Lewis structure for sulfur dichloride should show that two lone pairs of electrons are present on the sulfur atom.
These lone pairs of electrons are responsible for giving the molecule a bent molecular geometry, much like the two lone pairs of electrons present on the oxygen atom are responsible for giving the water molecule a bent geometry.
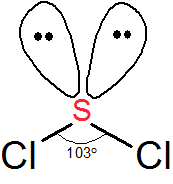
Consequently, the two dipole moments that arise from the difference in electronegativity that exists between the sulfur and the two chlorine atoms will not cancel each other out.
The
So remember, the polarity of a molecule depends on two things
- the polarity of the bonds
#-># you must have polar bonds in order to be able to talk about a polar molecule- the shape of the molecule
#-># symmetrical molecules are nonpolar regardless if they have polar bonds or not
Therefore, you can say that a polar molecule must
- have polar bonds
- be assymetrical

