Ozonolysis
Key Questions
-
An ozonide is the 1,2,4-trioxolane structure that is formed when ozone reacts with an alkene,
The first intermediate in the reaction is called a molozonide.
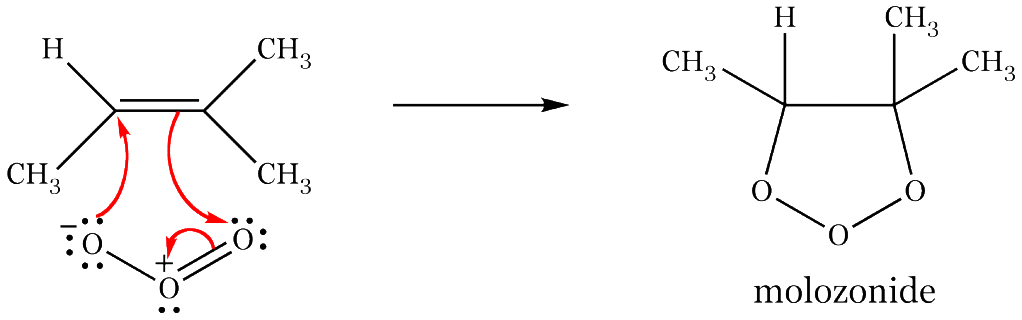
A molozonide is a 1,2,3-trioxolane (tri ="three"; oxa = "oxygen"; olane = "saturated 5-membered ring").
The molozonide is unstable. It rapidly converts in a series of steps to an ozonide.

An ozonide is a 1,2,4-trioxolane. It rapidly decomposes in water to form carbonyl compounds such as aldehydes and ketones.
The video below shows the formation of the molozonide and ozonide intermediates as part of the mechanism.
-
Ozonolysis does not tell you about any stereochemistry there may have been in the original alkene.
Reductive ozonolysis converts an alkene into a pair of carbonyl compounds.
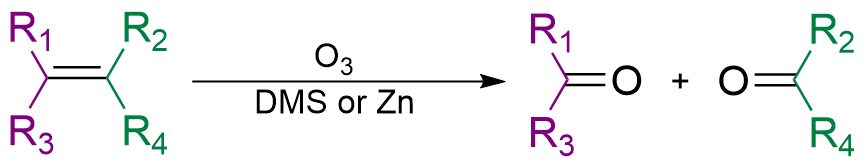
If the R groups are different, we can have cis/trans or E/Z stereochemistry.

If R₂ and R₄ are the groups with higher priority, 1 is a Z alkene. 2 is an E alkene.
But both give the same ozonolysis products.
You cannot distinguish between cis-but-2-ene and trans-but-2-ene by reductive ozonolysis.
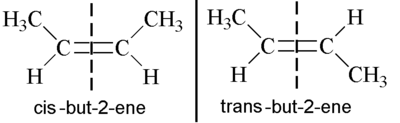
They both give acetaldehyde as the only ozonolysis product.