Atomic Orbitals and Periodic Table Relationships
Key Questions
-
Answer:
There are many types of atomic orbital (
#"s, p, d, f, g, h"# , …), but only the first four are occupied in the ground state of an atom.Explanation:
Quantum numbers
Two quantum numbers determine the type of orbital.
The principal quantum number,
#n# , determines the size of the orbital.The secondary quantum number,
#l# , determines the shape.#"s"# orbitalsFor each value of
#n# , there is one orbital for which#l = 0# .These orbitals are spheres.
The higher the value of
#n# , the larger the sphere.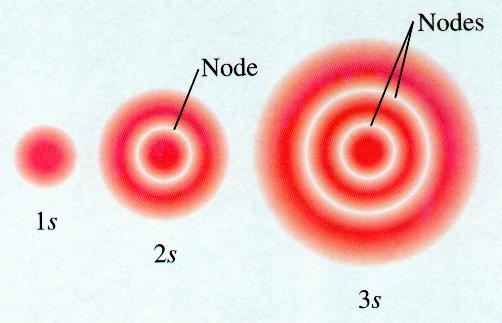
The spheres like nested shells separated by nodes — areas where there is no electron density.
#"p"# orbitalsWhen
#n > 1# ,#l# can have any value up to#n"-1"# .When
#l = 1# , the orbital is called a#"p"# orbital.A
#"p"# orbital looks like a dumbbell.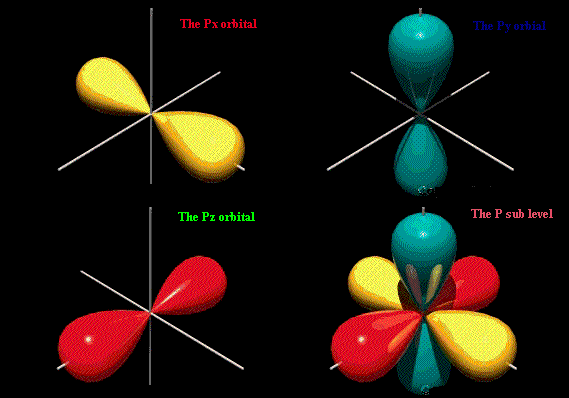
There are three types of
#"p"# orbital.Each points in a different direction.
#"d"# orbitalsWhen
#n = 3# , we can have orbitals with#l = 2# .These are called
#"d"# orbitals, and there are five of them.
One looks like a dumbbell with a doughnut around the middle.
The other four
#"d"# orbitals look like four-leaf clovers with the leaves pointing in different directions.#"f"# orbitalsWhen
#n = 4# , we can have orbitals with#l = 3# .These are called
#"f"# orbitals, and there are seven of them.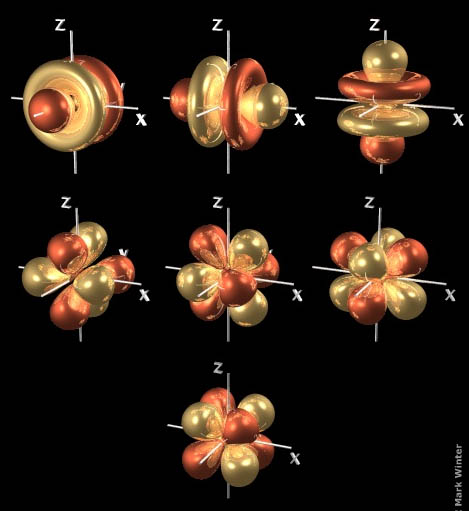
Three of the orbitals look like a dumbbell with two donuts around the middle.
The other four orbitals look like a bundle of eight balloons tied together and pointing to the corners of a cube.