Question #d2617
1 Answer
The histidine participates in the catalysis by way of acid-base proton transfers called a charge relay system.
We can eliminate the metal ion option, because histidine contains no metal.
The histidine participates in a number of H-bonding interactions, but that is not one of the options.
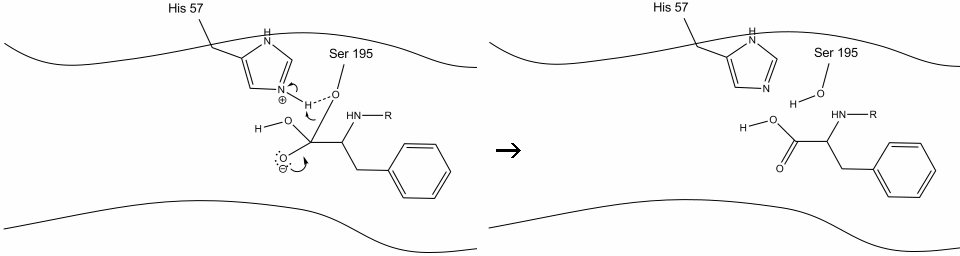
Step 1
After the target enters the active site, the O atom in the OH group on serine performs a nucleophilic attack on the carbonyl carbon of a peptide, forming a tetrahedral oxyanion intermediate.
The histidine acts as a base and accepts the proton from the OH group of serine.
Step 2
The oxyanion re-forms the carbonyl group and the amide bond is cleaved to form an acyl-enzyme intermediate.
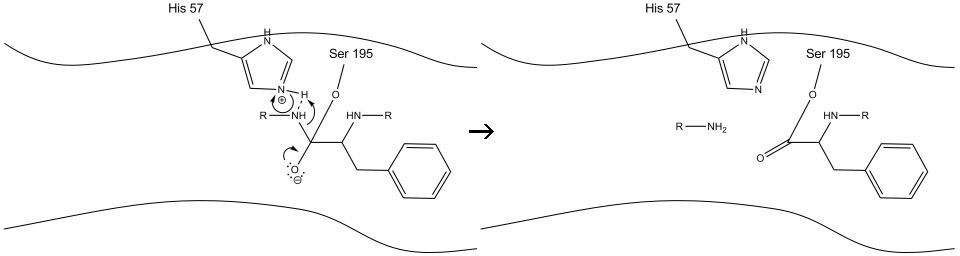
The newly-formed N-terminus takes back the proton from the protonated histidine.
Step 3
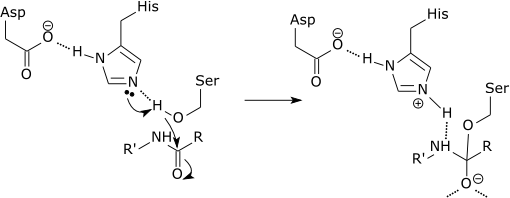
Water molecules now enter and hydrogen bond with the histidine.
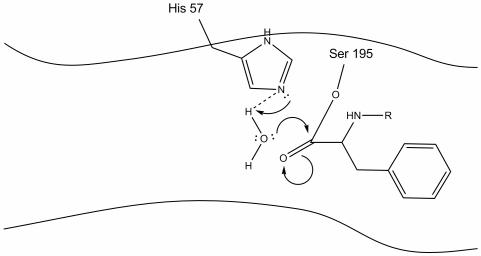
Step 4
The water now makes a nucleophilic attack on the carbonyl carbon of the acyl-enzyme intermediate and forms another oxyanion.
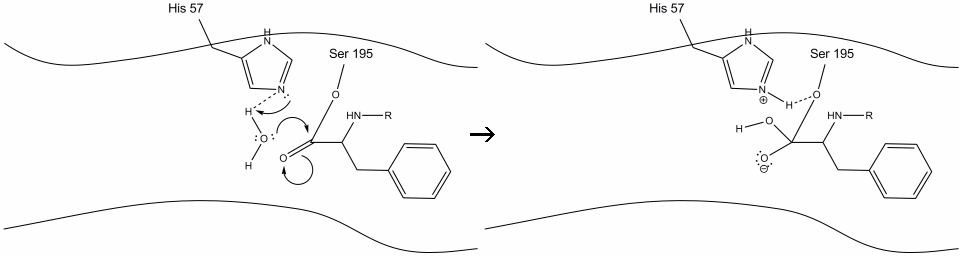
The histidine accepts a proton from the water and forms a hydrogen bond to the serine.
Step 5
The serine oxygen deprotonates the histidine nitrogen and re-forms the original enzyme.