Question #2c01b
1 Answer
!! LONG ANSWER !!
The hybridization of an atom that's part of a molecule can be easily determined by inspecting the Lewis structure of said molecule.
More specifically, orbital hybridization can be determined by counting the regions of electron density, which can be bonds to other atoms or lone pairs, that surround a certain atom.
The number of regions of electron density determines the steric number of an atom, which in turn determines orbital hybridization. So, let's take a look at your molecules.
- Ethanol,
#C_2H_6OH#
The Lewis structure for ethanol looks like this
 )
)
Look at the carbon atom located on the far left of the molecule. Notice that it is bonded to four other atoms (3 hydrogens and 1 carbon), and that it has no lone pairs present.
Since each bond counts as one region of electron density, its steric number will be equal to 4. Therefore, it will have 4 hybrid orbitals, which implies that it is
The same can be said for the central carbon atom. It is also bonded to four other atoms (2 hydrogens, 1 carbon, and 1 oxygen), so its steric number will be equal to 4.
The central carbon atom will thus be
- Methanal,
#CH_2O#
The Lewis structure for methanal is
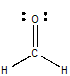
This time, the central carbon atom is surrounded by three bonds (double and triple bonds count as 1 region of electron density) and has no lone pairs present, which means that its steric number will be equal to 3.
This corresponds to an
The oxygen atom in only bonded to one other atom, the central carbon, but has 2 lone pairs of electrons present. Since each lone pair counts as 1 region of electron density, its steric number will be equal to 3 as well.
Once again, this corresponds to an
- Methanimine,
#CH_3N#
The Lewis structure for methanimine looks like this
 )
)
The carbon on the far left is bonded to four other atoms and has no lone pairs present, so its steric number is equal to 4 and it is
The carbon on the far right is only bonded to two other atoms and has no lone pairs present, so its teric number will be equal to 2. This corresponds to an

