Question #18423
1 Answer
Orbital hybridization actually tells you s-character.
Explanation:
Before deciding how much s-character a hybrid orbital has, you must first determine the type of hybrid orbital you're dealing with.
To do that, draw the Lewis structures of the molecules.
Let's start with xenon tetrafluoride,
The xenon atom will be the central atom of the molecule and it will form a single bond with each of the four fluorine atoms, which will have 3 lone pairs of electrons attached.
The remaining 4 valence electrons will be placed as lone pairs on the xenon atom.
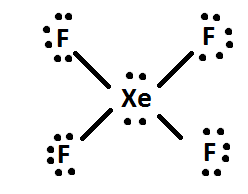
Now take a look at how many regions of electron density surround the central atom. This number, which is called steric number, will tell you what the hybridization of the central atom is.
The xenon atom is bonded to 4 fluorine atoms and has 2 lone pairs present, which means that it is surrounded by a total of 6 regions of electron density.
This means that it must use 6 hybrid orbitals
- one s-orbital
- three p-orbitals
- two d-orbitals
The central atom is
In this case, you have one s-orbital and six total orbitals, which means that you get
Now take a look at the carbonate ion,

