Question #dd0d4
1 Answer
Mar 13, 2017
The hybridization of
Explanation:
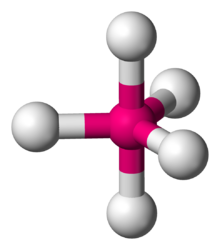
The Lewis structure of
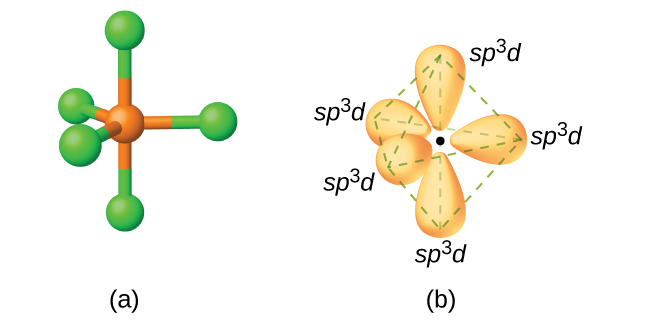
According to VSEPR theory, a molecule with five bonding pairs must have both a trigonal bipyramidal electron geometry and molecular geometry.
The
It has a
These orbitals are hybridized to form five