Which of the following molecules has five σ bonds and one π bond?
(a) #"C"_2"H"_6#
(b) #"C"_2"H"_3"Cl"#
(c) #"C"_2"H"_2"Cl"#
(d) #"C"_2"H"_4#
(a)
(b)
(c)
(d)
1 Answer
Apr 18, 2017
The correct answer is d)
Explanation:
a)

Ethane has six σ bonds formed by the overlap of
b)
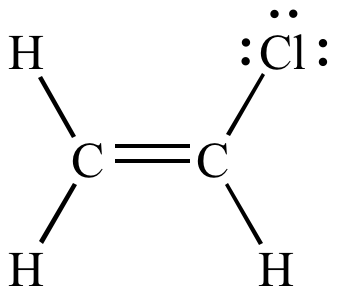
This compound has only three σ bonds (the
c)
This compound does not exist.
d)
Ethylene has (i) four σ bonds formed by the overlapping of
(ii) one σ bond formed by overlapping of two


