How do you determine which orbitals are used in hybridization?
2 Answers
Yes. For general chemistry, we just count the number of electron groups around the central atom, and assume that the orbitals used are in order of angular momentum
"NORMAL", GENERAL CHEMISTRY WAY
We assume an ordering of
#s, p, p, p, d, d, d, d, d, f, f, f, f, f, f, f#
or
#sp^3d^5f^7# .
For example, in

(You can think of it as the central atom "anticipating" future bonds it can possibly make.)
Or, in
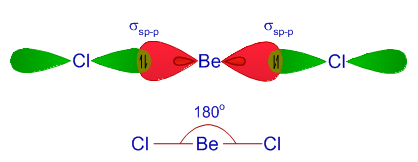
ABREVIATED VERSION:
Determine geometry of
(Is this linear of a derived structure?)
#V = 1Sn + 2Cl = 1(4) + 2(7) = 18#
#S = 2Cl = 2(8) = 16#
Parent = BPr + NBPr = 2 + 1 = 3 => Trigonal Planar Parent
=> Derived Geometry
=> This is also the number of hybrid orbitals needed. =>
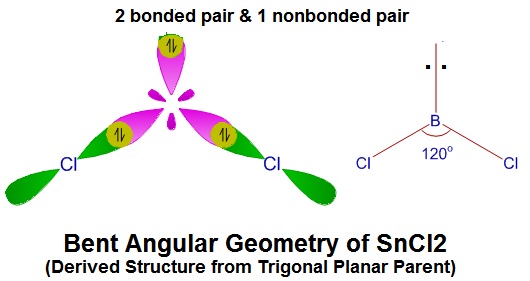
Determine the Geometry of
(Is this Trigonal Planar or a Derived Structure?)
#V = 1N + 3H = 1(5) + 3(1) = 8#
#S = 3H = 3(2) = 6#
BPr + NBPr = 3 + 1 = 4 => Tetrahedral Parent
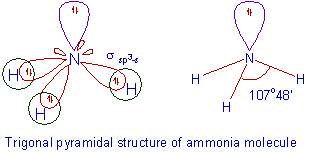
Determine the Geometry of
(Is this a Tetrahedral Geometry or a Derived Structure?)
#V = 1I + 4Cl + 1e^- = 1(7) + 4(7) + 1 = 36#
#S = 4Cl = 4(8) = 32#
BPr + NBPr = 4 + 2 = 6 => Octahedral Parent
 )
)
STURCTURES WITH MULTIPLE BONDS
Determine the Geometry of
(The Lewis Structure shows this to be
BPr = 3
NBPr = 0
BPr + NBPr = 3 => Trigonal Planar Geometry about Carbon. This means that the structure is composed of


