How do I determine the hybridization and bonding in ammonia?
1 Answer
Here's how to do it.
Explanation:
You draw the Lewis structure (Step 1) and use VSEPR theory to determine its electron domain geometry as tetrahedral and its molecular shape (Step 2) as a trigonal pyramid.
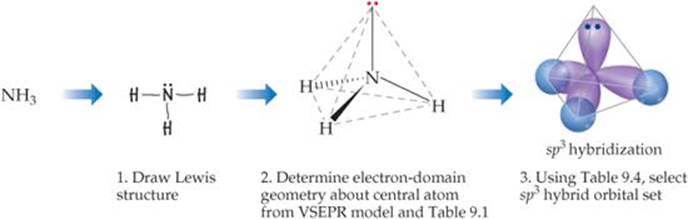
Then you consult a table like the one below.
(Adapted from SlideShare)
You find that the
The
That shows that the
However, the molecular orbitals include both the
In the diagram above, the bond-line drawing shows that the

